A Review of Hydrothermal Carbonization of Carbohydrates for Carbon Spheres Preparation
Abstract
Carbon spheres have attracted a great deal of attention due to their applications as super capacitors, catalyst supports, and adsorbents. Carbon spheres can be prepared with controlled size and with oxygenated functional groups on the surface by the hydrothermal carbonization. The further processed products have a high surface area and high thermal stability. Among various methods for fabrication of carbon spheres, the hydrothermal carbonization is favored because of its mild operating conditions. In addition, hydrothermal carbonization can synthesize micro or nano scale carbon spheres environmentally friendly without employing organic solvents, surfactants, or catalysts. In this review, we present the effects of process parameters, structural characteristics of carbon spheres, possible formation mechanisms of carbon spheres, and applications in catalysis.
Citation: Li, R., & Shahbazi, A. (2015). A Review of Hydrothermal Carbonization of Carbohydrates for Carbon Spheres Preparation. Trends in Renewable Energy, 1(1), 43-56. doi:http://dx.doi.org/10.17737/tre.2015.1.1.009
Keywords
Full Text:
Full Text (PDF)References
Mi, C., and Chen, W. (2014). Highly nanoporous carbon microflakes from discarded dental impression materials. Materials Letters, 114, 129-131. DOI: 10.1016/j.matlet.2013.10.010
White, R. J. (2015). Porous Carbon Materials from Sustainable Precursors, Royal Society of Chemistry
Chen, J., Lang, Z., Xu, Q., Hu, B., Fu, J., Chen, Z., and Zhang, J. (2013). Facile Preparation of monodisperse carbon spheres: Template-free construction and their hydrogen storage properties. ACS Sustainable Chemistry & Engineering, 1(8), 1063-1068. DOI: 10.1021/sc400124b
Zhang, K., Zhao, Q., Tao, Z., and Chen, J. (2013). Composite of sulfur impregnated in porous hollow carbon spheres as the cathode of Li-S batteries with high performance. Nano Research, 6(1), 38-46. DOI: 10.1007/s12274-012-0279-1
Zhang, Z., Xiao, F., Xi, J., Sun, T., Xiao, S., Wang, H., Wang, S., and Liu, Y. (2014). Encapsulating Pd Nanoparticles in Double-Shelled Graphene@Carbon Hollow Spheres for Excellent Chemical Catalytic Property. Scientific reports, 4, Article number: 4053. DOI: 10.1038/srep04053
Alazemi, A. A., Etacheri, V., Dysart, A., Stacke, L.-E., Pol, V., and Sadeghi, F. (2015). Ultrasmooth Submicron Carbon Spheres as Lubricant Additives for Friction and Wear Reduction. ACS Applied Materials & Interfaces, 7(9), 5514-5521. DOI: 10.1021/acsami.5b00099
Tien, B., Xu, M., and Liu, J. (2010). Synthesis and electrochemical characterization of carbon spheres as anode material for lithium-ion battery. Materials Letters, 64(13), 1465-1467. DOI: 10.1016/j.matlet.2010.03.061
Tooming, T., Thomberg, T., Romann, T., Palm, R., Jänes, A., and Lust, E. (2013). Carbon materials for supercapacitor application by hydrothermal carbonization of D-glucose. IOP Conf. Ser.: Mater. Sci. Eng, 49, 012020. DOI: 10.1088/1757-899X/49/1/012020
Auer, E., Freund, A., Pietsch, J., and Tacke, T. (1998). Carbons as supports for industrial precious metal catalysts. Applied Catalysis A: General, 173(2), 259-271. DOI: 10.1016/S0926-860X(98)00184-7
Chen, L.-F., Liang, H.-W., Lu, Y., Cui, C.-H., and Yu, S.-H. (2011). Synthesis of an Attapulgite Clay@Carbon Nanocomposite Adsorbent by a Hydrothermal Carbonization Process and Their Application in the Removal of Toxic Metal Ions from Water. Langmuir, 27(14), 8998-9004. DOI: 10.1021/la2017165
Qian, H.-s., Han, F.-m., Zhang, B., Guo, Y.-c., Yue, J., and Peng, B.-x. (2004). Non-catalytic CVD preparation of carbon spheres with a specific size. Carbon, 42(4), 761-766. DOI: 10.1016/j.carbon.2004.01.004
Serp, P., Feurer, R., Kalck, P., Kihn, Y., Faria, J., and Figueiredo, J. (2001). A chemical vapour deposition process for the production of carbon nanospheres. Carbon, 39(4), 621-626. DOI: 10.1016/S0008-6223(00)00324-9
Joo, J. B., Kim, P., Kim, W., Kim, J., Kim, N. D., and Yi, J. (2008). Simple preparation of hollow carbon sphere via templating method. Current Applied Physics, 8(6), 814-817. DOI: 10.1016/j.cap.2007.04.038
Ryoo, R., Joo, S. H., and Jun, S. (1999). Synthesis of Highly Ordered Carbon Molecular Sieves via Template-Mediated Structural Transformation. The Journal of Physical Chemistry B, 103(37), 7743-7746. DOI: 10.1021/jp991673a
Jin, Y. Z., Gao, C., Hsu, W. K., Zhu, Y., Huczko, A., Bystrzejewski, M., Roe, M., Lee, C. Y., Acquah, S., and Kroto, H. (2005). Large-scale synthesis and characterization of carbon spheres prepared by direct pyrolysis of hydrocarbons. Carbon, 43(9), 1944-1953. DOI: 10.1016/j.carbon.2005.03.002
Friedel, B., and Greulich-Weber, S. (2006). Preparation of Monodisperse, Submicrometer Carbon Spheres by Pyrolysis of Melamine–Formaldehyde Resin. small, 2(7), 859-863. DOI: 10.1002/smll.200500516
Titirici, M.-M., and Antonietti, M. (2010). Chemistry and materials options of sustainable carbon materials made by hydrothermal carbonization. Chemical Society Reviews, 39(1), 103-116. DOI: 10.1039/B819318P
Byrappa, K., and Adschiri, T. (2007). Hydrothermal technology for nanotechnology. Progress in Crystal Growth and Characterization of Materials, 53(2), 117-166. DOI: 10.1016/j.pcrysgrow.2007.04.001
Yao, C., Shin, Y., Wang, L.-Q., Windisch, C. F., Samuels, W. D., Arey, B. W., Wang, C., Risen, W. M., and Exarhos, G. J. (2007). Hydrothermal Dehydration of Aqueous Fructose Solutions in a Closed System. The Journal of Physical Chemistry C, 111(42), 15141-15145. DOI: 10.1021/jp074188l
Baccile, N., Laurent, G., Babonneau, F., Fayon, F., Titirici, M.-M., and Antonietti, M. (2009). Structural Characterization of Hydrothermal Carbon Spheres by Advanced Solid-State MAS 13C NMR Investigations. The Journal of Physical Chemistry C, 113(22), 9644-9654. DOI: 10.1021/jp901582x
Krishnamurthy, G., and Namitha, R. (2013). Synthesis of structurally novel carbon micro/nanospheres by low temperature-hydrothermal process. Journal of the Chilean Chemical Society, 58(3), 1930-1933. DOI: 10.4067/S0717-97072013000300030
Ramke, H. G., Blöhse, D., Lehmann, H. J., & Fettig, J. (2009). Hydrothermal carbonization of organic waste. Cossu, R., Diaz, LF, Stegman, R.(edts), Twelfth International Waste Management and Landfill Symphosium. Sardina: Pro., CISA pub.
Bröll, D., Kaul, C., Krämer, A., Krammer, P., Richter, T., Jung, M., ... & Zehner, P. (1999). Chemistry in supercritical water. Angewandte Chemie International Edition, 38(20), 2998-3014.
Libra, J. A., Ro, K. S., Kammann, C., Funke, A., Berge, N. D., Neubauer, Y., ... & Emmerich, K. H. (2011). Hydrothermal carbonization of biomass residuals: a comparative review of the chemistry, processes and applications of wet and dry pyrolysis. Biofuels, 2(1), 71-106.
Titirici, M.-M. (2013). Sustainable carbon materials from hydrothermal processes, Wiley Online Library.
Stranges, A. N. (1984). Friedrich Bergius and the Rise of the German Synthetic Fuel Industry. Isis, 75(4), 643-667. DOI: 10.2307/232411
Titirici, M.-M., Antonietti, M., and Baccile, N. (2008). Hydrothermal carbon from biomass: a comparison of the local structure from poly-to monosaccharides and pentoses/hexoses. Green Chemistry, 10(11), 1204-1212. DOI: 10.1039/B807009A
Wang, Q., Li, H., Chen, L., and Huang, X. (2001). Monodispersed hard carbon spherules with uniform nanopores. Carbon, 39(14), 2211-2214. DOI: 10.1016/S0008-6223(01)00040-9
Qi, X., Lian, Y., Yan, L., and Smith, R. L. (2014). One-step preparation of carbonaceous solid acid catalysts by hydrothermal carbonization of glucose for cellulose hydrolysis. Catalysis Communications, 57, 50-54. DOI: 10.1016/j.catcom.2014.07.035
Li, M., Li, W., and Liu, S. (2011). Hydrothermal synthesis, characterization, and KOH activation of carbon spheres from glucose. Carbohydrate Research, 346(8), 999-1004. DOI: 10.1016/j.carres.2011.03.020
Sevilla, M., and Fuertes, A. B. (2009). Chemical and structural properties of carbonaceous products obtained by hydrothermal carbonization of saccharides. Chemistry-A European Journal, 15(16), 4195-4203. DOI: 10.1002/chem.200802097
Mi, Y., Hu, W., Dan, Y., and Liu, Y. (2008). Synthesis of carbon micro-spheres by a glucose hydrothermal method. Materials Letters, 62(8), 1194-1196. DOI: 10.1016/j.matlet.2007.08.011
Yi, Z., Liang, Y., Lei, X., Wang, C., and Sun, J. (2007). Low-temperature synthesis of nanosized disordered carbon spheres as an anode material for lithium ion batteries. Materials Letters, 61(19), 4199-4203. DOI: 10.1016/j.matlet.2007.01.054
Zheng, M., Liu, Y., Xiao, Y., Zhu, Y., Guan, Q., Yuan, D., and Zhang, J. (2009). An easy catalyst-free hydrothermal method to prepare monodisperse carbon microspheres on a large scale. The Journal of Physical Chemistry C, 113(19), 8455-8459. DOI: 10.1021/jp811356a
Funke, A., and Ziegler, F. (2010). Hydrothermal carbonization of biomass: a summary and discussion of chemical mechanisms for process engineering. Biofuels, Bioproducts and Biorefining, 4(2), 160-177. DOI: 10.1002/bbb.198
Aydıncak, K., Yumak, T., Sınağ, A., & Esen, B. (2012). Synthesis and characterization of carbonaceous materials from saccharides (glucose and lactose) and two waste biomasses by hydrothermal carbonization. Industrial & Engineering Chemistry Research, 51(26), 9145-9152. DOI: 10.1021/ie301236h
Robbiani, Z. (2013). Hydrothermal carbonization of biowaste/fecal sludge: Conception and construction of a HTC prototype research unit for developing countries. Master's Thesis, Swiss Federal Institute of Technology in Zurich (ETHZ).
Demir-Cakan, R., Baccile, N., Antonietti, M., and Titirici, M.-M. (2009). Carboxylate-Rich Carbonaceous Materials via One-Step Hydrothermal Carbonization of Glucose in the Presence of Acrylic Acid. Chemistry of materials, 21(3), 484-490. DOI: 10.1021/cm802141h
Demir-Cakan, R., Hu, Y.-S., Antonietti, M., Maier, J., and Titirici, M.-M. (2008). Facile One-Pot Synthesis of Mesoporous SnO2 Microspheres via Nanoparticles Assembly and Lithium Storage Properties. Chemistry of materials, 20(4), 1227-1229. DOI: 10.1021/cm7031288
Yu, L., Falco, C., Weber, J., White, R. J., Howe, J. Y., and Titirici, M.-M. (2012). Carbohydrate-Derived Hydrothermal Carbons: A Thorough Characterization Study. Langmuir, 28(33), 12373-12383. DOI: 10.1021/la3024277
Falco, C., Baccile, N., and Titirici, M.-M. (2011). Morphological and structural differences between glucose, cellulose and lignocellulosic biomass derived hydrothermal carbons. Green Chemistry, 13(11), 3273-3281. DOI: 10.1039/C1GC15742F
Sun, X., and Li, Y. (2004). Colloidal Carbon Spheres and Their Core/Shell Structures with Noble-Metal Nanoparticles. Angewandte Chemie International Edition, 43(5), 597-601. DOI: 10.1002/anie.200352386
Yin, S., and Tan, Z. (2012). Hydrothermal liquefaction of cellulose to bio-oil under acidic, neutral and alkaline conditions. Applied Energy, 92, 234-239. DOI: 10.1016/j.apenergy.2011.10.041
Berge, N. D., Ro, K. S., Mao, J., Flora, J. R. V., Chappell, M. A., and Bae, S. (2011). Hydrothermal Carbonization of Municipal Waste Streams. Environmental Science & Technology, 45(13), 5696-5703. DOI: 10.1021/es2004528
Sevilla, M., Maciá-Agulló, J. A., & Fuertes, A. B. (2011). Hydrothermal carbonization of biomass as a route for the sequestration of CO2: Chemical and structural properties of the carbonized products. Biomass and Bioenergy, 35(7), 3152-3159. DOI: 10.1016/j.biombioe.2011.04.032
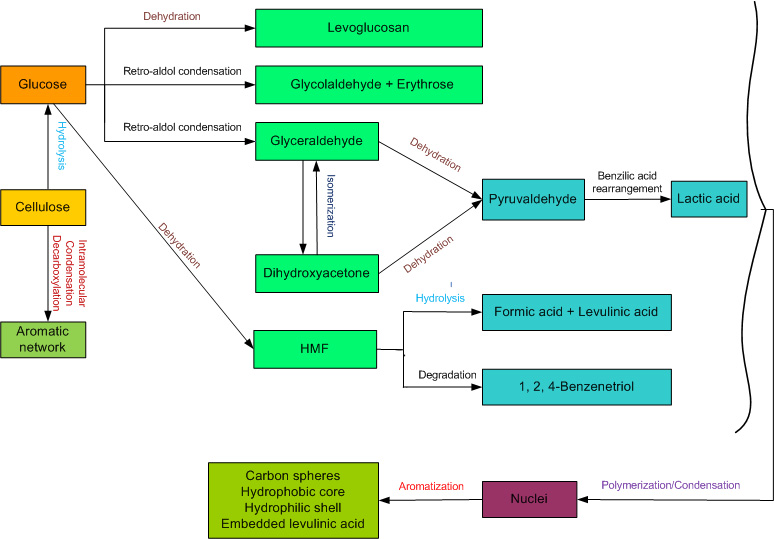
Chheda, J. N., Román-Leshkov, Y., & Dumesic, J. A. (2007). Production of 5-hydroxymethylfurfural and furfural by dehydration of biomass-derived mono-and poly-saccharides. Green Chemistry, 9(4), 342-350. DOI: 10.1039/B611568C
Xiang, Q., Lee, Y., and Torget, R. (2004). Kinetics of glucose decomposition during dilute-acid hydrolysis of lignocellulosic biomass. Applied Biochemistry and Biotechnology, 115(1-3), 1127-1138. DOI: 10.1385/abab:115:1-3:1127
Antal, M. J., Mok, W. S., and Richards, G. N. (1990). Mechanism of formation of 5-(hydroxymethyl)-2-furaldehyde from D-fructose and sucrose. Carbohydrate Research, 199(1), 91-109. DOI: 10.1016/0008-6215(90)84096-D
Peterson, A. A., Vogel, F., Lachance, R. P., Fröling, M., Antal Jr, M. J., & Tester, J. W. (2008). Thermochemical biofuel production in hydrothermal media: a review of sub-and supercritical water technologies. Energy & environmental science, 1(1), 32-65. DOI: 10.1039/B810100K
Sasaki, M., Goto, K., Tajima, K., Adschiri, T., and Arai, K. (2002). Rapid and selective retro-aldol condensation of glucose to glycolaldehyde in supercritical water. Green Chemistry, 4(3), 285-287. DOI: 10.1039/b203968k
Srokol, Z., Bouche, A.-G., van Estrik, A., Strik, R. C., Maschmeyer, T., and Peters, J. A. (2004). Hydrothermal upgrading of biomass to biofuel; studies on some monosaccharide model compounds. Carbohydrate Research, 339(10), 1717-1726. DOI: 10.1016/j.carres.2004.04.018
Kabyemela, B. M., Adschiri, T., Malaluan, R., and Arai, K. (1997). Degradation Kinetics of Dihydroxyacetone and Glyceraldehyde in Subcritical and Supercritical Water. Industrial & Engineering Chemistry Research, 36(6), 2025-2030. DOI: 10.1021/ie960747r
Chen, L. (2011). Conversion of Glycerol to Lactic Acid under Low Corrosive Conditions with Homogeneous and Heterogeneous Catalysts. Master's Thesis, University of Tennessee
Asghari, F. S., and Yoshida, H. (2007). Kinetics of the Decomposition of Fructose Catalyzed by Hydrochloric Acid in Subcritical Water: Formation of 5-Hydroxymethylfurfural, Levulinic, and Formic Acids. Industrial & Engineering Chemistry Research, 46(23), 7703-7710. DOI: 10.1021/ie061673e
Möller, M., Nilges, P., Harnisch, F., & Schröder, U. (2011). Subcritical water as reaction environment: fundamentals of hydrothermal biomass transformation. ChemSusChem, 4(5), 566-579. DOI: 10.1002/cssc.201000341
Weingarten, R., Conner, W. C., and Huber, G. W. (2012). Production of levulinic acid from cellulose by hydrothermal decomposition combined with aqueous phase dehydration with a solid acid catalyst. Energy & Environmental Science, 5(6), 7559-7574. DOI: 10.1039/c2ee21593d
Luijkx, G. C. A., van Rantwijk, F., and van Bekkum, H. (1993). Hydrothermal formation of 1,2,4-benzenetriol from 5-hydroxymethyl-2-furaldehyde and d-fructose. Carbohydrate Research, 242(0), 131-139. DOI: 10.1016/0008-6215(93)80027-C
Chuntanapum, A., and Matsumura, Y. (2009). Formation of Tarry Material from 5-HMF in Subcritical and Supercritical Water. Industrial & Engineering Chemistry Research, 48(22), 9837-9846. DOI: 10.1021/ie900423g
Salak Asghari, F., and Yoshida, H. (2006). Acid-Catalyzed Production of 5-Hydroxymethyl Furfural from d-Fructose in Subcritical Water. Industrial & Engineering Chemistry Research, 45(7), 2163-2173. DOI: 10.1021/ie051088y
Scallet, B. L., and Gardner, J. H. (1945). Formation of 5-Hydroxymethylfurfural from D-Glucose in Aqueous Solution. Journal of the American Chemical Society, 67(11), 1934-1935. DOI: 10.1021/ja01227a017
Patil, S. K., and Lund, C. R. (2011). Formation and growth of humins via aldol addition and condensation during acid-catalyzed conversion of 5-hydroxymethylfurfural. Energy & Fuels, 25(10), 4745-4755. DOI: 10.1021/ef2010157
LaMer, V. K., and Dinegar, R. H. (1950). Theory, production and mechanism of formation of monodispersed hydrosols. Journal of the American Chemical Society, 72(11), 4847-4854. DOI: 10.1021/ja01167a001
Li, T., Shen, J., Huang, S., Li, N., and Ye, M. (2014). Hydrothermal carbonization synthesis of a novel montmorillonite supported carbon nanosphere adsorbent for removal of Cr (VI) from waste water. Applied Clay Science, 93-94, 48-55. DOI: 10.1016/j.clay.2014.02.015
Yang, R., Zhao, W., Zheng, J., Zhang, X., and Li, X. (2010). One-Step Synthesis of Carbon-Coated Tin Dioxide Nanoparticles for High Lithium Storage. The Journal of Physical Chemistry C, 114(47), 20272-20276. DOI: 10.1021/jp107396a
Zhao, N., Wu, S., He, C., Wang, Z., Shi, C., Liu, E., and Li, J. (2013). One-pot synthesis of uniform Fe3O4 nanocrystals encapsulated in interconnected carbon nanospheres for superior lithium storage capability. Carbon, 57, 130-138. DOI: 10.1016/j.carbon.2013.01.056
Lou, X. W., Chen, J. S., Chen, P., and Archer, L. A. (2009). One-Pot Synthesis of Carbon-Coated SnO2 Nanocolloids with Improved Reversible Lithium Storage Properties. Chemistry of materials, 21(13), 2868-2874. DOI: 10.1021/cm900613d
Chen, J. S., Zhang, Y., and Lou, X. W. (2011). One-Pot Synthesis of Uniform Fe3O4 Nanospheres with Carbon Matrix Support for Improved Lithium Storage Capabilities. ACS Applied Materials & Interfaces, 3(9), 3276-3279. DOI: 10.1021/am201079z
Yu, G., Sun, B., Pei, Y., Xie, S., Yan, S., Qiao, M., ... & Zong, B. (2010). Fe x O y@ C spheres as an excellent catalyst for Fischer− Tropsch synthesis. Journal of the American Chemical Society, 132(3), 935-937. DOI: 10.1021/ja906370b
Cheng, J., Wang, Y., Teng, C., Shang, Y., Ren, L., and Jiang, B. (2014). Preparation and characterization of monodisperse, micrometer-sized, hierarchically porous carbon spheres as catalyst support. Chemical Engineering Journal, 242, 285-293. DOI: 10.1016/j.cej.2013.12.089
Makowski, P., Cakan, R. D., Antonietti, M., Goettmann, F., and Titirici, M.-M. (2008). Selective partial hydrogenation of hydroxy aromatic derivatives with palladium nanoparticles supported on hydrophilic carbon. Chemical Communications(8), 999-1001. DOI: 10.1039/B717928F
Shen, W., Zhu, Y., Dong, X., Gu, J., and Shi, J. (2005). A new strategy to synthesize TiO2-hollow spheres using carbon spheres as template. ChemInform, 34(40), no. DOI: 10.1002/chin.200540213
Wang, F.-L., Pang, L.-L., Jiang, Y.-Y., Chen, B., Lin, D., Lun, N., Zhu, H.-L., Liu, R., Meng, X.-L., and Wang, Y. (2009). Simple synthesis of hollow carbon spheres from glucose. Materials Letters, 63(29), 2564-2566. DOI: 10.1016/j.matlet.2009.09.008
DOI: http://dx.doi.org/10.17737/tre.2015.1.1.009
Refbacks
- There are currently no refbacks.
Copyright (c) 2015 Rui Li and Abolghasem Shahbazi

This work is licensed under a Creative Commons Attribution 4.0 International License.
 This work is licensed under a Creative Commons Attribution 4.0 License.
This work is licensed under a Creative Commons Attribution 4.0 License.Copyright @2014-2025 Trends in Renewable Energy (ISSN: 2376-2136, online ISSN: 2376-2144)
